The other metals in Group I are lithium potassium rubidium and francium. Beryllium and magnesium Both elements appear on the same group IIand therefore have the most similar chemical properties.

Potassium Principal Compounds And Reactions With Other Elements Britannica
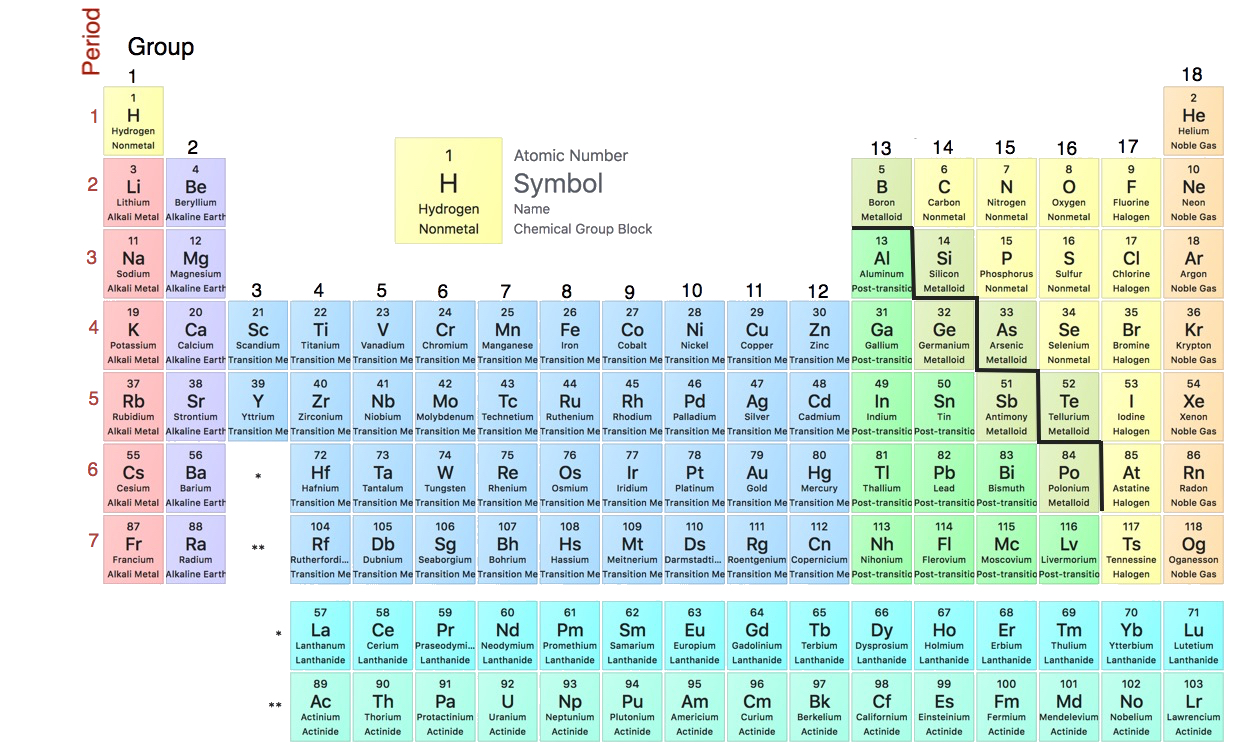
The columns are called groups.

. 6 How does metal have similar properties to Lithium. 9 What do Beryllium and lithium have in common. For an element two electrons can not have the same a Principal quantum numbers b Azimuthal quantum numbersc Magnetic quantum numbers d All qu.
Get the answers you need now. This is because their atoms have the same number of electrons in the highest occupied energy level. Consequently they have similar behavior when it comes to bonding and reacting with other substances.
3 Do lithium and beryllium have similar properties. Lithium and sodium have similar chemical properties to potassium as they are in the same group of the Periodic Table. Sodium and rubidium have the same properties with potassium.
Two elements that have properties similar to those of the element potassiumk. Sodium and Potassium C. Which element listed should have chemical properties similar to fluorine F ALi BSi CBr DNe Br along with F a halogen column 7 of the periodic table Look at the periodic table.
Correct answer to the question Which two elements have similar properties and 8 electrons in their outermost shells. Two elements that have properties similar to those of the element sodium are Potassium K and Lithium Li because they are in the same group. For example Sodium potassium lithium rubidium and caesium have similar chemical properties.
As you descend the Group the metal the element becomes more reactive. Im pretty sure. Iodine and Bromine D.
4 What elements have similar properties. 3 phosphorus and sulfur 4 potassium and strontium Answer. 8 What is the chemical properties of lithium.
When you compare the chemical properties of these elements lithium sodium potassium rubidium cesium and francium what youll notice is that they are all remarkably similar. 7 How does sodium have similar properties to Lithium. The information that can be included in a periodic table is symbols and names of elements along with the information about the structure of their.
The elements potassium and sodium have similar chemical properties because they have the same number of valence electrons 1. This is because sodium potassium and rubidium are in the same column that is they are all in group one of periodic table. The correct answer 1.
Zoreianmcfarlane zoreianmcfarlane 29032021 Chemistry. 5 Which element has properties like lithium quizlet. To those of kryptonkr.
Both elements sodium Na and potassium K lie in the Group 1 column of the Periodic Table which contains the members of the Alkali. Therefore Elements with similar chemical properties have D the same number of electrons in an outermost shell as the elements in the same group only have the same number of electrons in the outermost shell. The elements in the first column of the Periodic Table other than hydrogen are known as Group 1A metals or alkali metals.
Both sodium and potassium are in the first vertical column of the periodic charge making them Group 1 alkali metals. Elements in the same group in the periodic table have similar chemical properties. Alkali metals are extremely reactive.
From the list below which element has properties similar to potassium. All are alkali metals hence have the same properties. Predict the group of elements and their number of valence.
What information can be included in a periodic table. L i lithium and K potassium both belong to first group alkali metals and. Arsenic and Antimony B.
For example dropping even a small amount of pure sodium metal in room temperature water creates an intensely strong reaction that produces high temperatures and generates hydrogen gas.
Potassium Chemical Element Reaction Water Uses Elements Examples Metal Gas Number
0 Comments